Patients should follow the instructions for use in their kit for additional test instructions and updated return shipping information. Please contact CDI’s dedicated customer service team at 888-258-5966 or customerservice@commdx.com with questions or concerns.
Direct Breath Test Ordering Now Available for Patients
Welcome to the CDI Patient Resource Center.
Contact our dedicated patient services team for additional support.
SIBO & IMO
10 Tube Lactulose
(Adult GI)
SIBO & IMO
10 Tube Glucose
(Adult GI)
SIBO & IMO
6 Tube Lactulose
(Pediatric GI)
FAQs and Billing & Insurance
SIBO is the accumulation of an excessive amount of gut bacteria in the small intestine (at least 100,000 bacteria per ml of fluid). While bacteria naturally exist throughout the digestive tract, with the highest concentrations of bacteria in the colon, a healthy individual should have relatively low levels of bacteria present in the small intestine. Any condition which impairs the normal transit or motion of the small intestine can increase the likelihood of getting SIBO, including lack of adequate stomach acid, damage to the intestine by toxins, or a decrease in the speed at which the small intestine transfers material to the colon. In the U.S., some research studies have demonstrated that up to 80% of the IBS population, or 36 million individuals, suffer from SIBO, and some researchers even hypothesize a connection between SIBO and common skin disorders like acne and rosacea.
Hydrogen and methane breath testing is based on the concept that bacteria can enter and proliferate in the small intestine and produce trace gases that are not generated by any normal function in the human body. By measuring the levels of these trace gases (hydrogen and methane) in the breath using gas chromatography, the test can aid in the diagnosis of small intestinal bacterial overgrowth (SIBO) and other gastrointestinal disorders like sugar malabsorption
We provide a pre-paid, pre-addressed shipping label in each kit. Patients will simply take the completed, sealed test kit and return it to us using the shipping label provided.
When the kit arrives at our laboratory, it will be analyzed within 1 business day. Kits that arrive on Saturdays or Holidays will be analyzed the following business day. Results will be sent to your practice via a secure fax system, secure email, or your office can set up an account with us to receive results via our HIPAA-compliant web-based reporting system.
Results will be sent to your provider within 24-48 hours of receiving your test kit at our laboratory.
CDI may not know that a test is invalid until it is fully processed by the lab. Failure to follow the Instructions For Use located in each patient’s test kit can lead to an invalid test result. Patient may still be billed for the test if the test is invalid due to patient error.
An invalid breath test means that CDI was not able to provide a valid result. Here is a list of reasons why your breath may be invalid:
- Breath test arrived after the two-week analysis timeframe
- Invalid Baseline Sample, click here to learn more (link to invalid baseline FAQ)
- Incorrect collection timing
- Invalid samples
- Did not label test tubes
- Test kit was expired
- Non-CDI kit components were used
- Substrate was not ingested at the correct time
- Samples were collected across multiple days
- Multiple damaged septa
Please note that the specific reason for an invalid result is provided in the report. Please contact your provider for more information. CDI will bill patients when the result is an “Invalid” due to poor patient prep, patient misuse, or other failures to comply with Instructions for Use. In that scenario, however, CDI also offers those patients a complimentary second test if the patient completes payment for their original test.
Invalid baselines are caused by the patient not taking a proper sample for the first tube. CDI may not know that a test is invalid until it is fully processed by the lab. Patients may still be billed for the test if the test is invalid due to patient error. As all of our results are based on the baseline sample, and we follow the North American Consensus on Breath Testing, CDI is not able to provide a valid result when the baseline sample is not collected properly. Additional information is provided on your result report provided to your doctor that explains how your provider can use the results to help either form an interpretation or recommend retesting.
CDI will bill patients when the result is an “Invalid” due to an invalid baseline sample, however, CDI also offers patients a complimentary second test if the patient completes payment for their original test.
CDI offers interpretation assistance to providers only for all of our diagnostic test kits. Please contact your provider for information relating to your results. Please note that CDI can only discuss the following with your provider: parameters of our test, test methods, most common results and instrumentation questions. Patient symptoms may vary, so it’s important to discuss your symptoms and correlations with your provider directly. You can find an explanation of the 6 most common results for hydrogen & methane breath tests here. CDI follows guidance from the North American Consensus on Breath Testing for all of our hydrogen & methane breath tests. A summary of the consensus can be found here, and the full consensus from the American Journal of Gastroenterology can be found here.
Only the following foods are permitted during the 12-hour preparatory diet period:
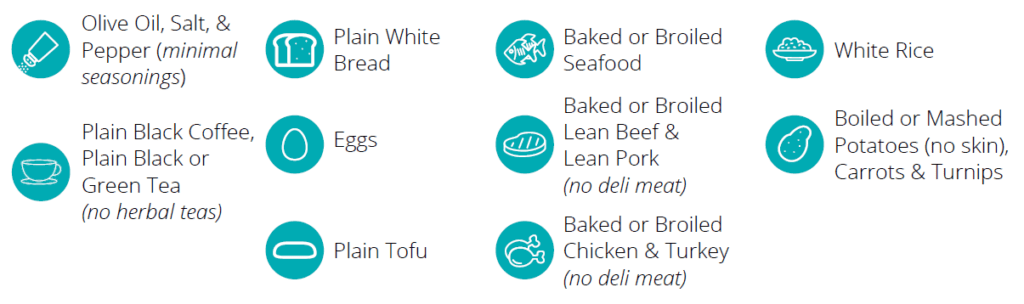
NOTE: All other foods not listed above are not allowed during the 12-hour preparatory diet. Foods such as, but not limited to: alcohol, dairy, beans, wheat, grains, fruits and vegetables, and high-fiber containing foods are not allowed. Be sure to consult the Instructions for Use included in your kit.
Yes. A 12-hour fast is required prior to all breath tests. Water can be consumed during this time and during the test.
- Discontinue the use of any antibiotics for 4 weeks prior to taking the test.
- Discontinue the use of any laxatives and/or promotility drugs for 1 week prior to taking the test.
- Do not smoke or vape for at least 24 hours prior to the test, or any time during the test.
- Do not sleep or exercise for at least 1 hour prior to taking the test or at any time during the test.
If you remembered to collect the baseline sample within 15 minutes of consuming the substrate, the test can be continued as normal. Be sure to collect the second sample 15 minutes after consuming the substrate. If the baseline sample was collected after 15 minutes, the test needs to be repeated.
If the event was within 15-30 minutes after consuming the substrate, the test needs to be repeated. If the event was more than 30 minutes after consuming the substrate, the test can be continued as normal.
Yes, you must drink the entire substrate mixture, quickly, for the test to be valid. If you do not drink the entire mixture, the test must be repeated.
No. Eating at any point during the test will make the test results invalid. The test must be repeated.
If either the times or sample numbers were recorded, then we can process your test. If you do not record the time of the sample, we will include a note to your health care provider with your test results. Your health care provider will determine whether to accept the test results that were not labeled with times for each sample. If neither the time nor sample numbers were recorded, the test will not be analyzed.
Glucose is absorbed by the digestive tract so it will not be able to detect SIBO that is further along in the small intestine (proximal jejunum/ileum). However, because of this, glucose is considered to be more sensitive than lactulose, providing less false positives with colonic bacteria.
Lactulose is not absorbed by the digestive tract and will travel through the entire gastrointestinal tract, ultimately providing a complete depiction of all intestinal segments, including the colon (large intestine).
In the United States, lactulose contains a 10-gram dose of lactulose with less than 1.6-grams of galactose, less than 1.2-grams of lactose, and 1.2- grams of other sugars. If you have a sensitivity to any of the sugars listed, please consult your healthcare provider/nutritionist for a possible alternative substrate he/she can prescribe. (i.e. glucose)
Outside the United States, lactulose contains a 10-gram dose of lactulose with more than 5-grams of lactose/galactose/epilactose. If you have a sensitivity to any of the sugars listed, please consult your healthcare provider/nutritionist for a possible alternative substrate he/she can prescribe. (i.e. glucose)
Glucose is 100% glucose sugar. If you are sensitive and/or have diabetes (Type I or II), please consult with your healthcare provider if this substrate is right for you.
Fructose is 100% fructose sugar.
Lactose is 100% lactose sugar.
Sucrose is 100% sucrose sugar.
Your breath samples are stable for up to two weeks and across a wide temperature range (-4°F to 104°F). Samples received after two weeks of collection will be invalid.
We consider any samples with a Carbon Dioxide (CO2) concentration of less than 2.00% to be an invalid sample.
If there are any components missing from any test kit, we will send out a new test kit to you or health care provider. Please contact our Customer Service Department at 1-888-258-5966 in this instance.
If you need to take multiple tests, you may not take more than one daily. Please wait at least 24 hours between taking each test, though you may wait longer. The full 24-hour preparatory period, 12 hours of following the restricted diet followed by twelve hours of fasting, must be adhered to before each test.
For any patient that has tested positive for COVID-19, CDI has adopted a corporate policy whereby you should not administer and return your test kit, and CDI will not accept samples from any known COVID-19 positive patient, until thirty days (30) have passed following a negative follow-up test for COVID-19 from your healthcare provider.
We are implementing this policy in an abundance of caution in order to provide for the safest possible environment for our patients as well as our employees and clinical staff. We thank you for your cooperation with our policies, we wish you a speedy recovery and good health, and we look forward to working with you in the near future to help diagnose your gastrointestinal symptoms.
Breath Tests
CDI will submit a claim on the patient’s behalf to commercial insurance, Medicare or Tricare. Insurance may cover some or all of the test depending on the patient’s insurance plan and benefits. In the event the patient’s insurance provider denies the insurance claim, or if the patient has not met the deductible or has a coinsurance or co-pay, or if for any reason the insurance does not cover the full amount of the test, the patient is responsible to pay CDI for products and services received.
CDI does not accept any Medicaid plans: therefore any Medicaid patient taking a test will be responsible for the full cost of the test. CDI offers convenient payment plans and financial hardship programs for qualifying patients. Patients may pay up front via check sent with the kit or credit card. The maximum out-of-pocket cost per test is $249 for patients that pay promptly in accordance with CDI patient billing policies and programs. For an updated list of in-network providers, please visit www.commdx.com/insurance.
- Breath Test FAQs
-
What is Small Intestinal Bacterial Overgrowth (SIBO)?
SIBO is the accumulation of an excessive amount of gut bacteria in the small intestine (at least 100,000 bacteria per ml of fluid). While bacteria naturally exist throughout the digestive tract, with the highest concentrations of bacteria in the colon, a healthy individual should have relatively low levels of bacteria present in the small intestine. Any condition which impairs the normal transit or motion of the small intestine can increase the likelihood of getting SIBO, including lack of adequate stomach acid, damage to the intestine by toxins, or a decrease in the speed at which the small intestine transfers material to the colon. In the U.S., some research studies have demonstrated that up to 80% of the IBS population, or 36 million individuals, suffer from SIBO, and some researchers even hypothesize a connection between SIBO and common skin disorders like acne and rosacea.
What is hydrogen and methane breath testing?Hydrogen and methane breath testing is based on the concept that bacteria can enter and proliferate in the small intestine and produce trace gases that are not generated by any normal function in the human body. By measuring the levels of these trace gases (hydrogen and methane) in the breath using gas chromatography, the test can aid in the diagnosis of small intestinal bacterial overgrowth (SIBO) and other gastrointestinal disorders like sugar malabsorption
How are patients’ kits returned to CDI?We provide a pre-paid, pre-addressed shipping label in each kit. Patients will simply take the completed, sealed test kit and return it to us using the shipping label provided.
What is the turnaround time for laboratory results?When the kit arrives at our laboratory, it will be analyzed within 1 business day. Kits that arrive on Saturdays or Holidays will be analyzed the following business day. Results will be sent to your practice via a secure fax system, secure email, or your office can set up an account with us to receive results via our HIPAA-compliant web-based reporting system.
How will I receive my results?Results will be sent to your provider within 24-48 hours of receiving your test kit at our laboratory.
I received an invalid test result, why am I still being billed for this test?CDI may not know that a test is invalid until it is fully processed by the lab. Failure to follow the Instructions For Use located in each patient’s test kit can lead to an invalid test result. Patient may still be billed for the test if the test is invalid due to patient error.
Why was my test invalid?An invalid breath test means that CDI was not able to provide a valid result. Here is a list of reasons why your breath may be invalid:
- Breath test arrived after the two-week analysis timeframe
- Invalid Baseline Sample, click here to learn more (link to invalid baseline FAQ)
- Incorrect collection timing
- Invalid samples
- Did not label test tubes
- Test kit was expired
- Non-CDI kit components were used
- Substrate was not ingested at the correct time
- Samples were collected across multiple days
- Multiple damaged septa
Please note that the specific reason for an invalid result is provided in the report. Please contact your provider for more information. CDI will bill patients when the result is an “Invalid” due to poor patient prep, patient misuse, or other failures to comply with Instructions for Use. In that scenario, however, CDI also offers those patients a complimentary second test if the patient completes payment for their original test.
My test result was invalid due to “Invalid Baseline Sample,” what does this mean and why am I still being billed for this test?Invalid baselines are caused by the patient not taking a proper sample for the first tube. CDI may not know that a test is invalid until it is fully processed by the lab. Patients may still be billed for the test if the test is invalid due to patient error. As all of our results are based on the baseline sample, and we follow the North American Consensus on Breath Testing, CDI is not able to provide a valid result when the baseline sample is not collected properly. Additional information is provided on your result report provided to your doctor that explains how your provider can use the results to help either form an interpretation or recommend retesting.
CDI will bill patients when the result is an “Invalid” due to an invalid baseline sample, however, CDI also offers patients a complimentary second test if the patient completes payment for their original test.
Can CDI help me understand my breath test results?CDI offers interpretation assistance to providers only for all of our diagnostic test kits. Please contact your provider for information relating to your results. Please note that CDI can only discuss the following with your provider: parameters of our test, test methods, most common results and instrumentation questions. Patient symptoms may vary, so it’s important to discuss your symptoms and correlations with your provider directly. You can find an explanation of the 6 most common results for hydrogen & methane breath tests here. CDI follows guidance from the North American Consensus on Breath Testing for all of our hydrogen & methane breath tests. A summary of the consensus can be found here, and the full consensus from the American Journal of Gastroenterology can be found here.
Are there dietary restrictions involved with taking the test?Only the following foods are permitted during the 12-hour preparatory diet period:

NOTE: All other foods not listed above are not allowed during the 12-hour preparatory diet. Foods such as, but not limited to: alcohol, dairy, beans, wheat, grains, fruits and vegetables, and high-fiber containing foods are not allowed. Be sure to consult the Instructions for Use included in your kit.
Is fasting required?Yes. A 12-hour fast is required prior to all breath tests. Water can be consumed during this time and during the test.
Are there any activity restrictions involved with taking the test?- Discontinue the use of any antibiotics for 4 weeks prior to taking the test.
- Discontinue the use of any laxatives and/or promotility drugs for 1 week prior to taking the test.
- Do not smoke or vape for at least 24 hours prior to the test, or any time during the test.
- Do not sleep or exercise for at least 1 hour prior to taking the test or at any time during the test.
What if I forgot to collect my baseline sample before consuming the substrate?If you remembered to collect the baseline sample within 15 minutes of consuming the substrate, the test can be continued as normal. Be sure to collect the second sample 15 minutes after consuming the substrate. If the baseline sample was collected after 15 minutes, the test needs to be repeated.
Will vomiting during the test affect my results?If the event was within 15-30 minutes after consuming the substrate, the test needs to be repeated. If the event was more than 30 minutes after consuming the substrate, the test can be continued as normal.
Do I have to drink the entire substrate?Yes, you must drink the entire substrate mixture, quickly, for the test to be valid. If you do not drink the entire mixture, the test must be repeated.
Can I eat during the test?No. Eating at any point during the test will make the test results invalid. The test must be repeated.
If I mislabel the tubes can my samples still be analyzed?If either the times or sample numbers were recorded, then we can process your test. If you do not record the time of the sample, we will include a note to your health care provider with your test results. Your health care provider will determine whether to accept the test results that were not labeled with times for each sample. If neither the time nor sample numbers were recorded, the test will not be analyzed.
What is the difference between glucose and lactulose substrates?Glucose is absorbed by the digestive tract so it will not be able to detect SIBO that is further along in the small intestine (proximal jejunum/ileum). However, because of this, glucose is considered to be more sensitive than lactulose, providing less false positives with colonic bacteria.
Lactulose is not absorbed by the digestive tract and will travel through the entire gastrointestinal tract, ultimately providing a complete depiction of all intestinal segments, including the colon (large intestine).
What are the ingredients to the sugar substrates?In the United States, lactulose contains a 10-gram dose of lactulose with less than 1.6-grams of galactose, less than 1.2-grams of lactose, and 1.2- grams of other sugars. If you have a sensitivity to any of the sugars listed, please consult your healthcare provider/nutritionist for a possible alternative substrate he/she can prescribe. (i.e. glucose)
Outside the United States, lactulose contains a 10-gram dose of lactulose with more than 5-grams of lactose/galactose/epilactose. If you have a sensitivity to any of the sugars listed, please consult your healthcare provider/nutritionist for a possible alternative substrate he/she can prescribe. (i.e. glucose)
Glucose is 100% glucose sugar. If you are sensitive and/or have diabetes (Type I or II), please consult with your healthcare provider if this substrate is right for you.
Fructose is 100% fructose sugar.
Lactose is 100% lactose sugar.
Sucrose is 100% sucrose sugar.
How long are my samples stable for?Your breath samples are stable for up to two weeks and across a wide temperature range (-4°F to 104°F). Samples received after two weeks of collection will be invalid.
When is a sample invalid?We consider any samples with a Carbon Dioxide (CO2) concentration of less than 2.00% to be an invalid sample.
What if my kit arrives and is missing components?If there are any components missing from any test kit, we will send out a new test kit to you or health care provider. Please contact our Customer Service Department at 1-888-258-5966 in this instance.
May I take more than one test on the same day?If you need to take multiple tests, you may not take more than one daily. Please wait at least 24 hours between taking each test, though you may wait longer. The full 24-hour preparatory period, 12 hours of following the restricted diet followed by twelve hours of fasting, must be adhered to before each test.
I tested positive for COVID-19. When can I take and return a CDI breath test?For any patient that has tested positive for COVID-19, CDI has adopted a corporate policy whereby you should not administer and return your test kit, and CDI will not accept samples from any known COVID-19 positive patient, until thirty days (30) have passed following a negative follow-up test for COVID-19 from your healthcare provider.
We are implementing this policy in an abundance of caution in order to provide for the safest possible environment for our patients as well as our employees and clinical staff. We thank you for your cooperation with our policies, we wish you a speedy recovery and good health, and we look forward to working with you in the near future to help diagnose your gastrointestinal symptoms.
- Billing & Insurance
-
Breath Tests
CDI will submit a claim on the patient’s behalf to commercial insurance, Medicare or Tricare. Insurance may cover some or all of the test depending on the patient’s insurance plan and benefits. In the event the patient’s insurance provider denies the insurance claim, or if the patient has not met the deductible or has a coinsurance or co-pay, or if for any reason the insurance does not cover the full amount of the test, the patient is responsible to pay CDI for products and services received.
CDI does not accept any Medicaid plans: therefore any Medicaid patient taking a test will be responsible for the full cost of the test. CDI offers convenient payment plans and financial hardship programs for qualifying patients. Patients may pay up front via check sent with the kit or credit card. The maximum out-of-pocket cost per test is $249 for patients that pay promptly in accordance with CDI patient billing policies and programs. For an updated list of in-network providers, please visit www.commdx.com/insurance.
