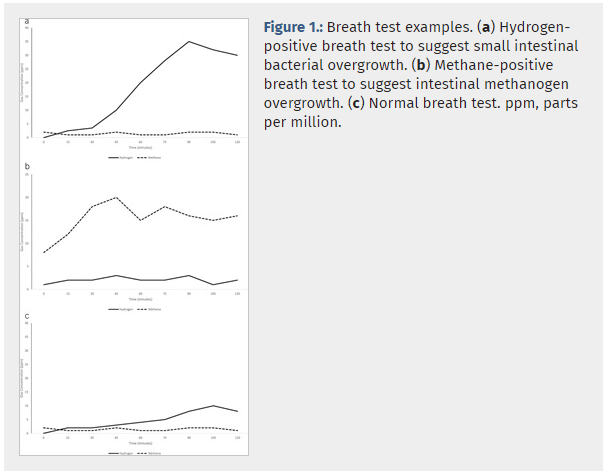
Small Intestinal Bacterial Overgrowth (SIBO) and Intestinal Methanogen Overgrowth (IMO) represent significant challenges in adult gastrointestinal health. These conditions are characterized by an atypical increase in bacteria or archaea in the small intestine, which can lead to a range of disruptive symptoms.
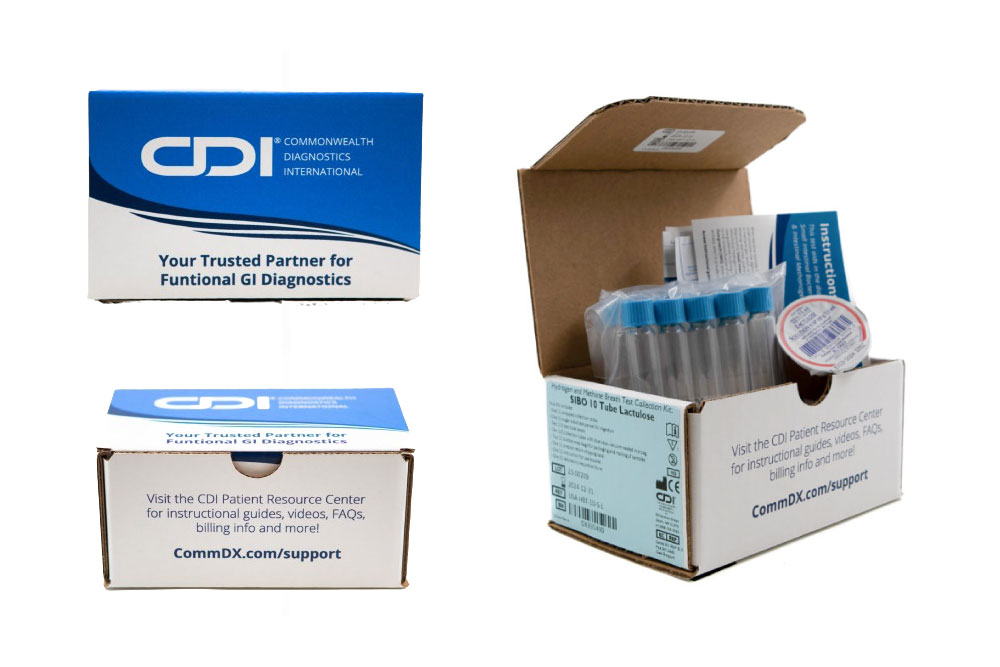
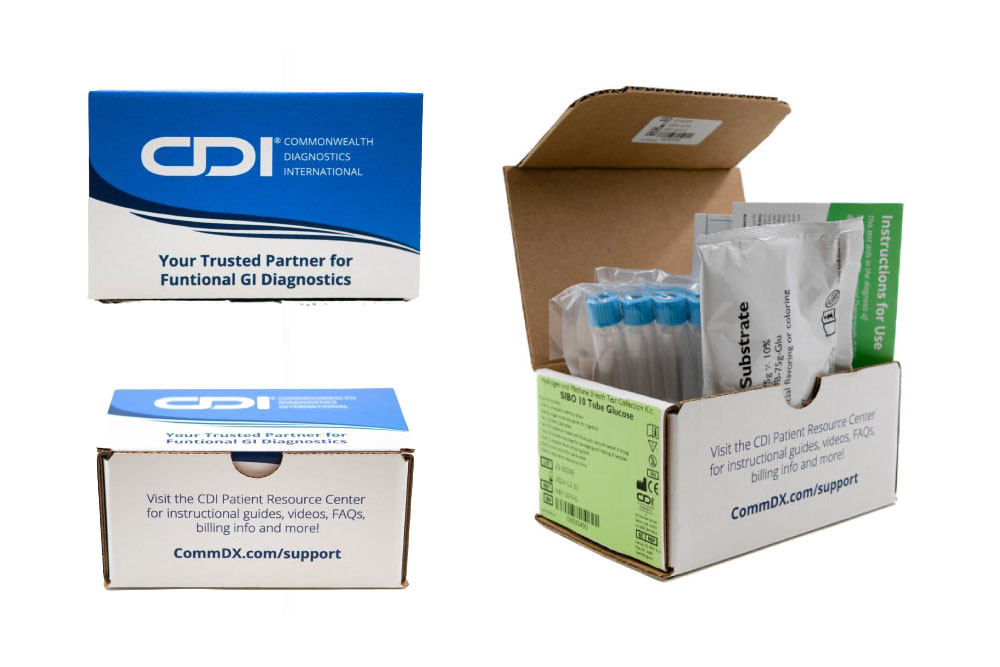
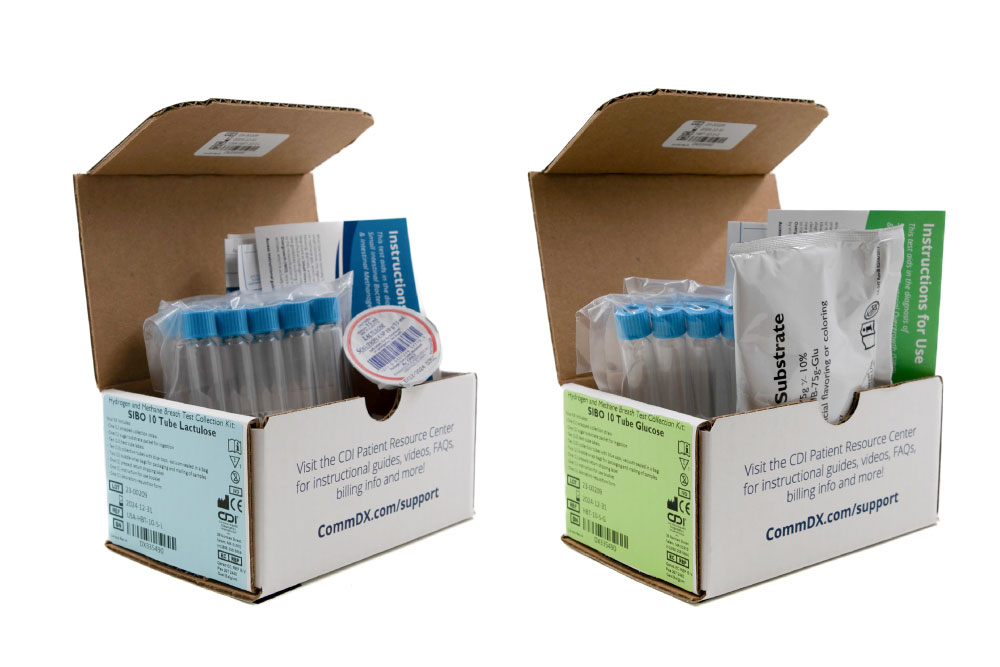
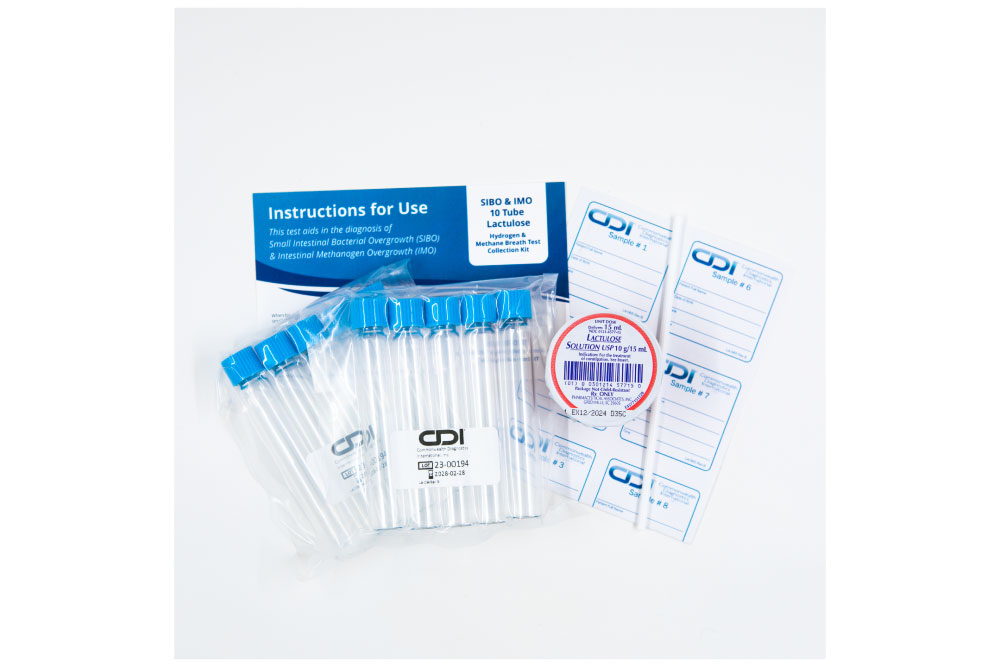
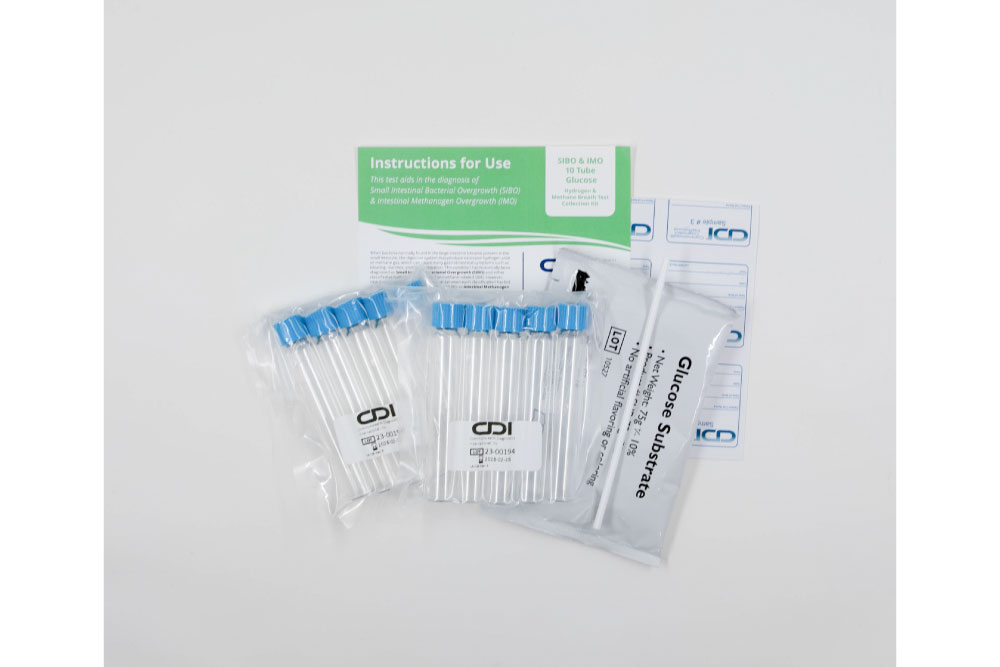
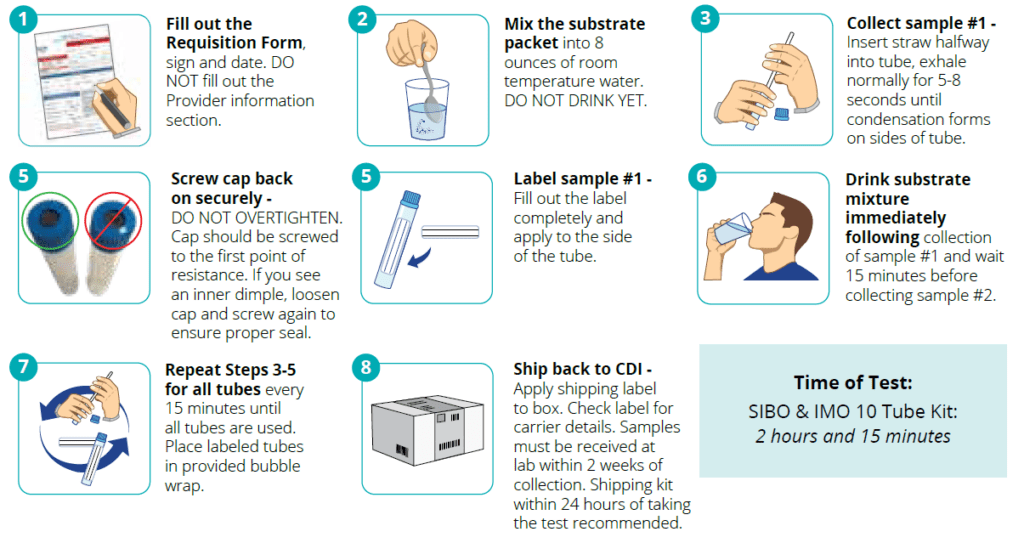
At CDI, we provide a specialized non-invasive Hydrogen and Methane 10 Tube Breath Test for SIBO & IMO, with either lactulose or glucose, crafted to meet the diagnostic needs of adult patients. This test not only pinpoints the presence of SIBO and IMO but also guides subsequent management strategies to address these complex conditions effectively.
Why Opt for CDI’s Adult SIBO & IMO Breath Test?
- Customized for Adults: This breath test is designed specifically to meet the diagnostic needs of adults, ensuring accuracy and reliability in detecting SIBO and IMO.
- Precision Diagnosis: Our method utilizes a 10-tube system to measure hydrogen and methane levels, providing a detailed profile of the gut’s microbial activity and enhancing diagnostic precision.
- Effective Symptom Relief: Early and accurate detection allows for personalized treatment plans that significantly mitigate symptoms like bloating, diarrhea, constipation, and abdominal pain, thereby enhancing patient well-being.
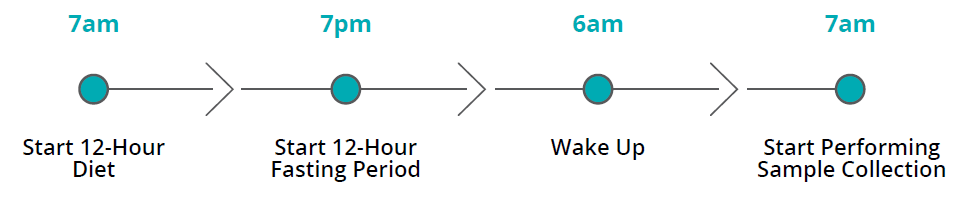
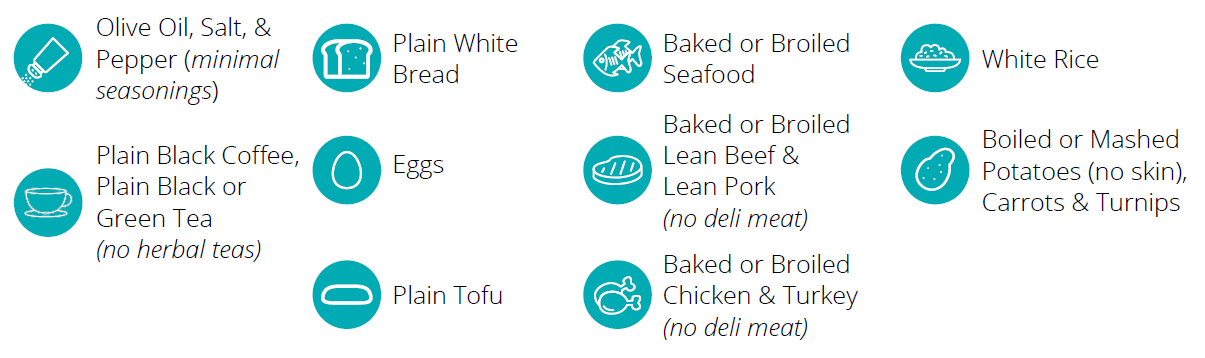
- Ease and Convenience: The breath test is non-invasive and can be conducted in the comfort of the home, ensuring patient convenience and compliance.
- Comprehensive Gastrointestinal Evaluation: In addition to SIBO and IMO, CDI offers diagnostic tests for carbohydrate malabsorption disorders such as fructose, lactose, and sucrose, ensuring a thorough assessment of gastrointestinal health.
- Research-Backed: Our adult breath test is supported by extensive scientific research and clinical validation, providing healthcare providers with a trustworthy diagnostic tool.
Our Hydrogen and Methane Breath Test for SIBO & IMO sets a new benchmark in gastrointestinal diagnostics for adults, supporting healthcare professionals in delivering precision-based, effective care. This advanced testing solution is part of CDI’s broader commitment to enhancing gastrointestinal health through innovative, patient-focused diagnostics.
Pediatric versions of our SIBO & IMO breath test kits are also available, ensuring that patients of all ages can access high-quality gastrointestinal diagnostics.