Small Intestinal Bacterial Overgrowth (SIBO) and Intestinal Methanogen Overgrowth (IMO) are complex conditions that can significantly impact the gastrointestinal health of pediatric patients. These disorders are characterized by an abnormal increase in the number of bacteria or specific types of methane-producing organisms in the small intestine, leading to uncomfortable and sometimes debilitating symptoms.
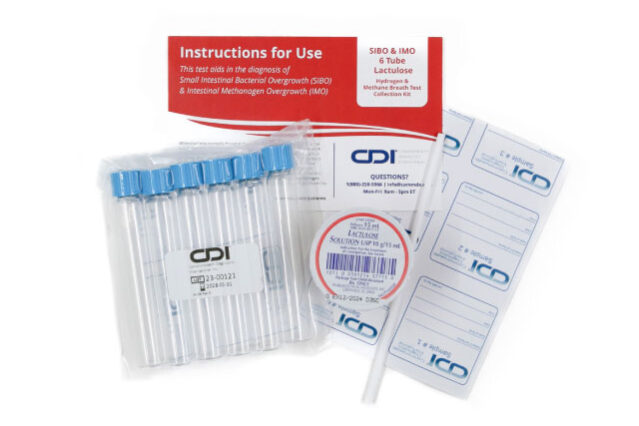
CDI is at the forefront of non-invasive diagnostic solutions with our Hydrogen and Methane 6 Tube Lactulose Breath Test for SIBO & IMO, designed specifically for pediatric care. This test offers a precise, patient-friendly way to identify the presence of SIBO and IMO, laying the groundwork for effective treatment strategies.
Why Opt for CDI’s Pediatric SIBO & IMO Breath Test?
- Tailored for Pediatric Needs: Our breath test is developed with the unique needs of pediatric patients in mind, ensuring a comfortable and stress-free testing experience.
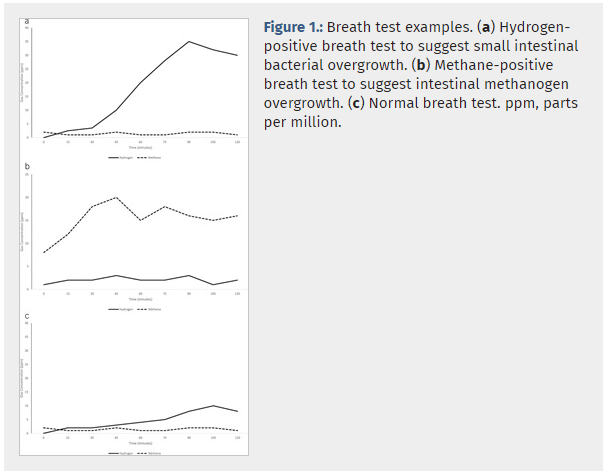
- Accurate Diagnosis: By measuring hydrogen and methane levels in response to a lactulose substrate, our test provides clear insights into the gut’s microbial environment, enabling accurate detection of SIBO and IMO.
- Symptom Management: Timely and precise diagnosis facilitates targeted treatments that can significantly alleviate symptoms such as bloating, diarrhea, and abdominal discomfort, improving the child’s overall quality of life.
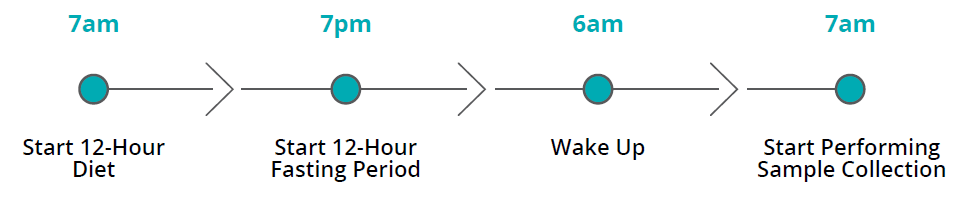
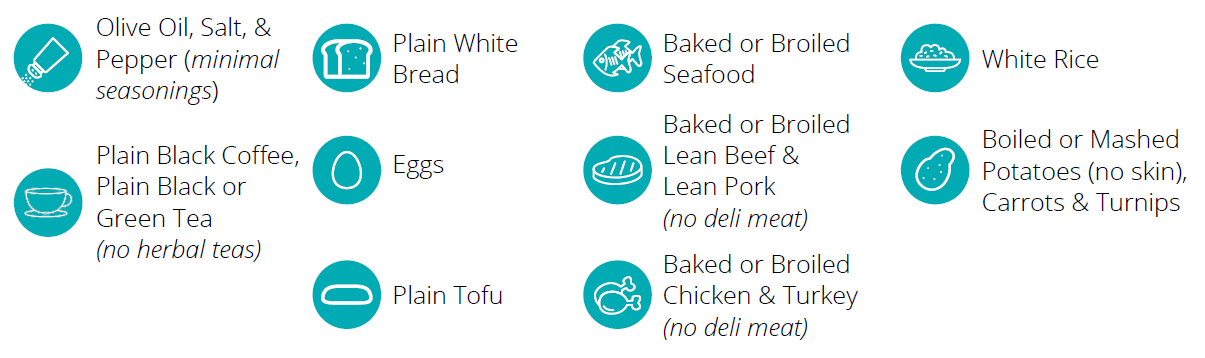
- Non-Invasive and Convenient: The simplicity of our breath test eliminates the need for invasive procedures, making it an ideal choice for children. The test can be administered at home, enhancing convenience for patients and their families.
- Comprehensive Care Approach: Beyond SIBO and IMO, CDI offers breath tests for fructose, lactose, and sucrose carbohydrate malabsorption disorders, providing a holistic overview of the child’s gastrointestinal health.
- Backed by Research: Our commitment to evidence-based care is reflected in the rigorous validation of our pediatric breath test, ensuring that healthcare providers have access to reliable diagnostic tools.
Embrace a new standard in pediatric gastrointestinal diagnostics with CDI’s Hydrogen and Methane 6 Tube Lactulose Breath Test for SIBO & IMO. This advanced testing solution empowers healthcare providers to deliver precise, effective care tailored to the needs of their youngest patients, paving the way for healthier, happier futures.
Discover the difference with CDI’s pediatric breath testing—where innovation meets patient-centered care in the pursuit of optimal GI health.