At-Home Breath Testing Program from CDI
Benefits to Healthcare Providers and their Patients
CDI’s at-home breath test kits for SIBO, IMO, and carbohydrate malabsorption disorders help physicians obtain accurate results to inform diagnostic and treatment plans. Our functional GI diagnostic test kits not only help support meaningful GI health outcomes, but also are easy to use, quick, safe, and cost-effective for patients.
Non-Invasive At-Home Hydrogen and Methane Breath Testing
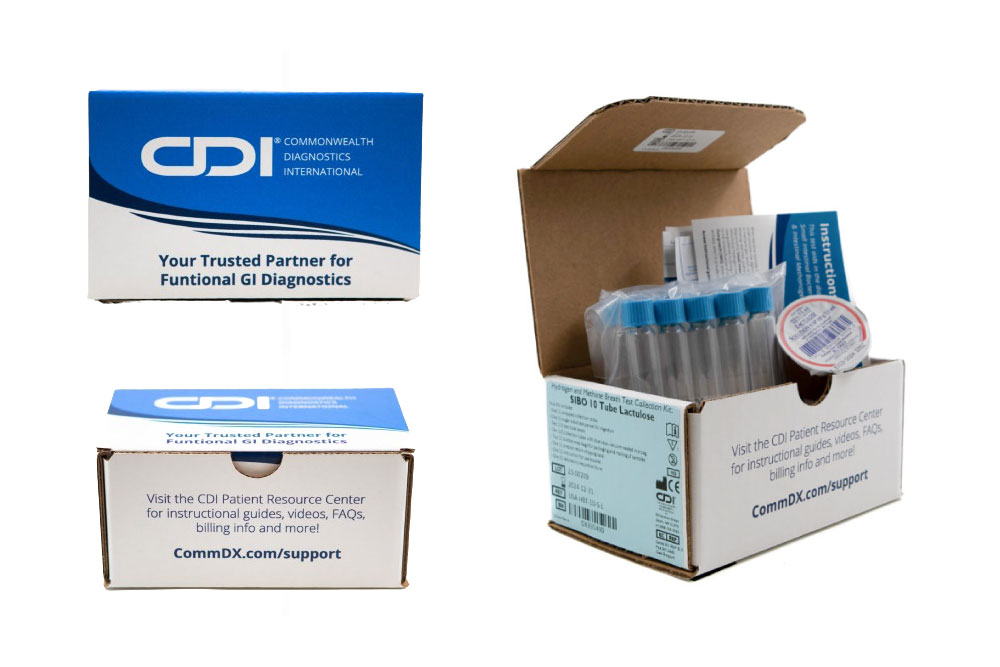
SIBO & IMO
(Adult GI)
SIBO and IMO in adult cases are common causes of functional bloating, diarrhea, constipation and suspected malabsorption.

SIBO & IMO
(Pediatric GI)
CDI’s non-invasive pediatric breath test, validated by rigorous research, can help identify SIBO and IMO in young patients.
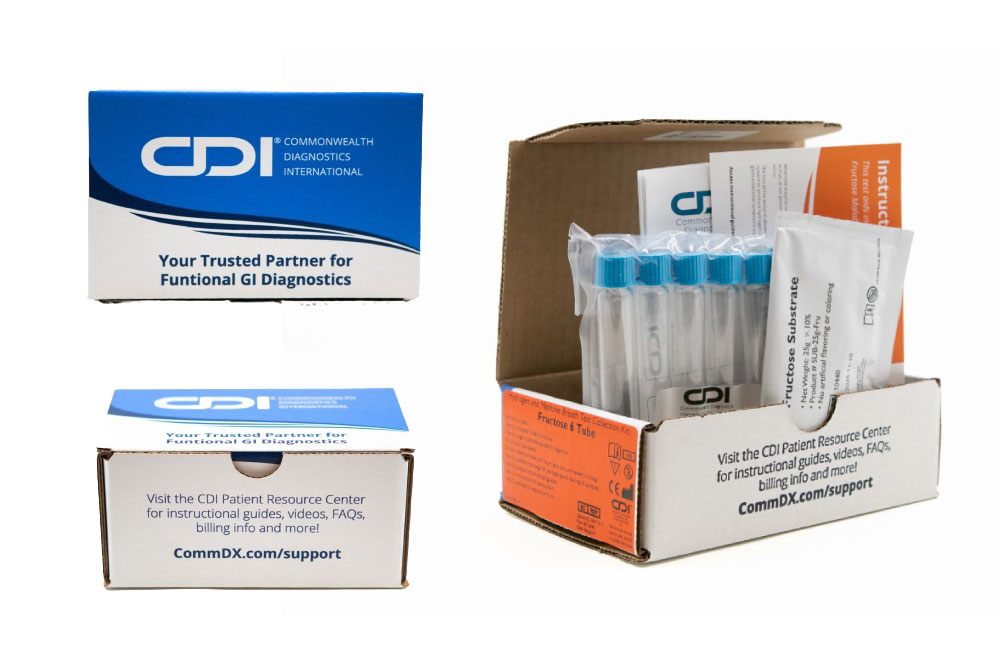
Fructose
Malabsorption
Fructose intolerance is a condition in which absorption of fructose is impaired by deficient fructose carriers in the small intestine’s enterocytes.

Lactose
Malabsorption
Lactose intolerance is the inability to digest the sugar lactose causing gastrointestinal symptoms such as flatulence, bloating, cramps, and diarrhea.
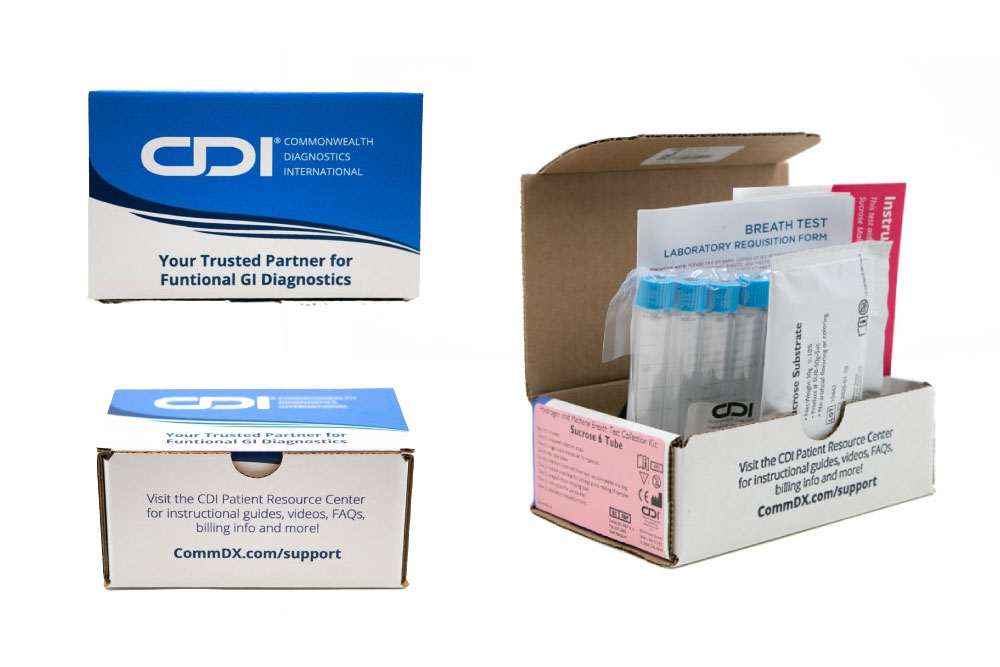
Sucrose
Malabsorption
Sucrose intolerance is the condition in which sucrase, an enzyme needed for proper metabolization of sucrose, is not produced in the small intestine.
Get free resources on trends and best practices for the diagnosis and treatment of functional GI disorders
Resources & Support
Visit our Resources pages for FAQs and additional materials for providers and patients.
Contact our dedicated customer service department at 888-258-5966 or customerservice@commdx.com for support.









