Hydrogen and methane breath testing plays a critical role in diagnosing functional gastrointestinal disorders such as SIBO, IMO, and carbohydrate malabsorption. A breath test is ordered to answer a specific clinical question and guide next steps in patient care. When a test is never completed or returned, that diagnostic pathway stops short.
Unreturned or unregistered breath test kits don’t just represent a logistical issue — they represent missed diagnoses and clinical context, delayed care, and unnecessary burden on both patients and practices. Ensuring every test ordered leads to a valid test result is an essential part of Breath Testing Done Right.
Unreturned Kits = Missed Diagnoses
Every breath test ordered reflects a patient who is seeking answers. When a kit goes unreturned, providers are left without objective data to confirm or rule out conditions like SIBO or IMO. This can lead to:
- Delayed diagnosis and prolonged symptoms
- Empiric or trial-and-error treatments
- Missing clinical context
- Additional office visits, labs, or procedures
- Frustration for patients who feel “stuck” without clarity
In functional GI care, where symptoms can be chronic and overlapping, objective breath test data is often the difference between uncertainty and a clear treatment plan.
The Hidden Operational Cost to Practices
Beyond clinical impact, unreturned kits also create inefficiencies:
- Cost of unused test kits and shipping
- Time spent distributing tests that never yield results
- Incomplete or unresolved patient charts
- Administrative burdens of chasing missing data
Over time, low return ratios quietly erode program efficiency and reduce the overall value of breath testing within a practice.
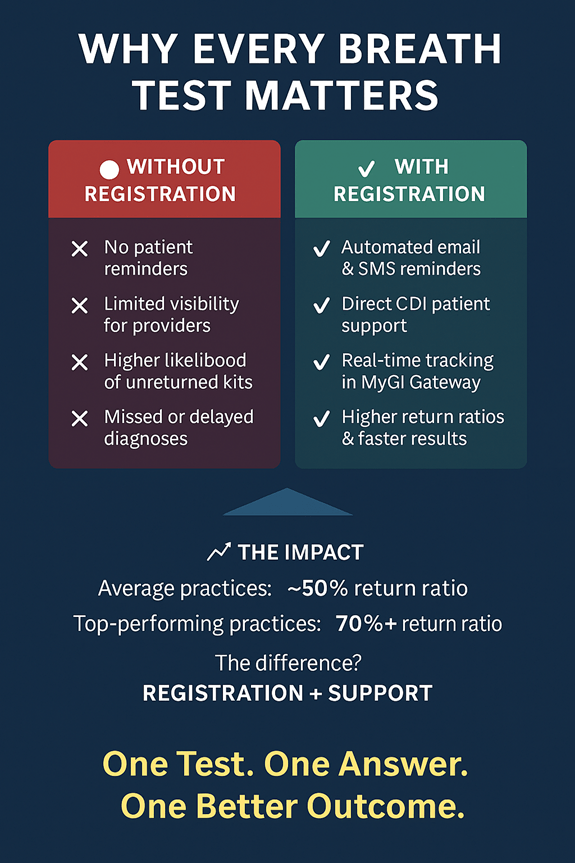
Kit Registration: The Single Most Important Step
Across CDI’s network, one factor consistently correlates with higher return ratios: kit registration.
When a kit is registered, CDI can:
- Send automated email and SMS reminders to patients
- Provide direct patient education and support
- Answer questions that often delay completion
- Track test status in real time for both patients and providers
Registration transforms a passive test into an actively supported diagnostic process.
| Ship-to-Practice Kits
For kits handed out in the office, compliance is highest when:
This simple step enables CDI to take over patient follow-up and significantly improves return rates. |
Ship-to-Patient Kits
For kits shipped directly to patients, compliance improves when:
This allows CDI to provide reminders, instructions, and real-time support throughout the testing process. |
What Top-Performing Practices Do Differently
Practices with return ratios consistently above 70%:
- Register kits at the point of distribution
- Provide complete patient contact information
- Use MyGI Gateway to monitor kit status
- Rely on CDI’s patient support team instead of internal follow-up
These workflows don’t add complexity — they remove friction.
Real-Time Visibility with MyGI Gateway
MyGI Gateway provides practices with a centralized view of:
- Kits ordered and shipped
- Registered vs. unregistered kits
- Tests in progress and completed results
This transparency allows practices to identify gaps before tests go missing proactively.
Closing the Loop Improves Patient Care
Every unreturned kit is a missed opportunity to deliver answers. Every completed test strengthens clinical decision-making, reduces delays, and improves patient trust.
At CDI, we view return ratio not as an operational metric — but as a clinical one. Ensuring every test matters means ensuring every test comes back.
If your team would benefit from workflow guidance, kit registration training, or a review of your program performance, our Provider Relations team is here to help.