According to the American College of Gastroenterology (ACG)’s new Clinical Guidelines for the Management of Irritable Bowel Syndrome (IBS), healthcare providers should focus on “a positive diagnostic strategy as compared to a diagnostic strategy of exclusion be used to improve time to initiating appropriate therapy.”
Additional peer-reviewed research that supports a diagnosis of inclusion also validates the presence of anti-vinculin and anti-cytolethal distending toxin B (CdtB) as blood-based biomarkers for IBS and separates IBS with diarrhea (IBS-D) from irritable bowel disease (IBD). Accordingly, the study describes a biomarker for IBS-D based on a possible pathophysiologic mechanism of post-infectious IBS and the subsequent adevelopment of autoantibodies to vinculin in the host.
CDI’s proprietary IBSchek® Capillary Collection Kit is based on the referenced scientific findings and was clinically validated in 2015 and revalidated more recently internationally. The non-invasive, at-home blood test is the most reliable diagnostic tool for post-infectious IBS in the market and directly supports ACG’s guidelines for a positive diagnostic strategy.
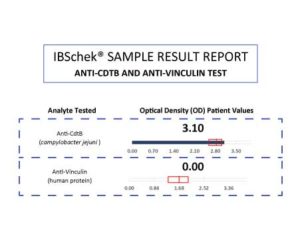 IBSchek results sent to healthcare providers and/or their patients utilize the two clinically validated antibodies – anti-CdtB and anti-vinculin – to indicate either a “Supported” or “Not Supported” test result for IBS-D or IBS-M.
IBSchek results sent to healthcare providers and/or their patients utilize the two clinically validated antibodies – anti-CdtB and anti-vinculin – to indicate either a “Supported” or “Not Supported” test result for IBS-D or IBS-M.
Here is a simple breakdown of the two possible test results for IBSchek:
- “Supportive” test results are confirmed when either antibody (anti-CdtB or anti-vinculin) level is greater than the indicated reference intervals. This suggests that there is a greater clinical significance for IBS-D or IBS-M and that the IBS may be due to previous gastroenteritis or bacterial infection.
- “Not Supportive” tests results are confirmed when both antibodies (anti-CdtB and anti-vinculin) levels are less than the indicated reference intervals. A “non supportive” test does not exclude IBS-D or IBS-M but may indicate that further studies are needed to rule out other causes for the patient’s gastrointestinal symptoms and/or complaints.
View an IBSchek Sample Result Report.
Healthcare providers should assess each patient’s clinical factors that may affect the interpretation of the test results and ensure that the test results correlate with the patient’s symptoms and other related findings for diagnostic and treatment purposes.
Learn more about CDI’s IBSchek Capillary Collection Kit.

