When patients present with symptoms related to functional gastrointestinal (GI) disorders or Disorders of the Gut-Brain Interaction (DGBI) like Small Intestinal Bacterial Overgrowth (SIBO), Intestinal Methanogen Overgrowth (IMO), and Carbohydrate Malabsorption, the recommended method of diagnosis is clinical breath testing.
Each breath test – overseen by providers at healthcare facilities or taken by patients in the comfort of their own home – presents an opportunity for a GI practice to capture clinically essential patient data and generate revenue for the service it provides its patients. Yet, the differences in upfront costs and revenue potential between point-of-care breath testing and at-home breath testing are significant.
This guide provides an in-depth look at these two breath test processes – point-of-care and at-home – including a pro-forma analysis of potential costs and revenue associated with both.
Download Full Digital Copy of
"A Pro-Forma Comparison of Breath Testing Models for Gastrointestinal Disorders"
In-Depth Look at Point-of-Care Breath Testing
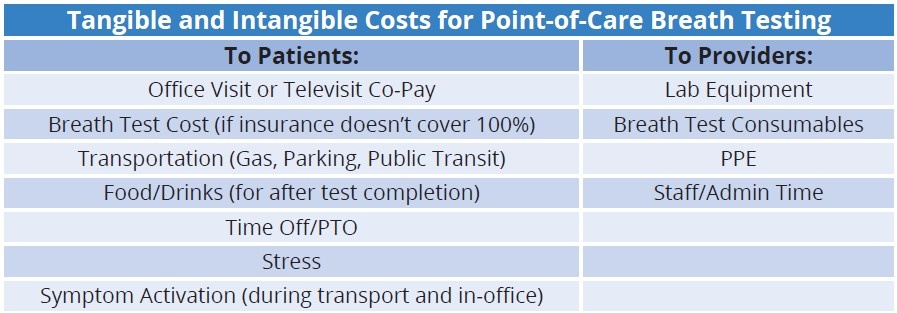
A standard session of breath testing in an office or healthcare setting consists of the patient drinking a substrate mixture and exhaling into a point-of-care (POC) breath collection device or breathalyzer instrument up to ten times – and in some cases more – at multiple intervals over two to three hours, depending on test type. A nurse or medical assistant typically helps administer the test in a medical exam room.
While there are few conveniences to in-office breath testing, the POC device model is dated, and it no longer provides the same clinical value or safety protocols necessary in a COVID-19 environment.
In-office breath testing requires patients to take time from work, school, and life to travel to and wait in the provider facility for an extended period—often three or more hours—to obtain the necessary breath samples. Additionally, administration and data collection are time-consuming and involve manual entry, leading to more significant data recording and transmission errors.
There is also a higher likelihood that in-office tests use inferior or dated instrumentation that does not meet the same rigorous standards as a Clinical Laboratory Improvement Amendments (CLIA) laboratory. The samples are usually neither run by professional lab technicians nor run in a CLIA-certified lab. This leads to open questions regarding quality assurance, daily instrument calibration, systems monitoring, and proficiency testing in the POC setting.
Finally, disinfection of devices, patient area, equipment, and PPE must occur between each patient, increasing costs and severely limiting the number of patient tests administered each day. This snowballs into longer wait times for diagnosis and treatment for patients suffering from DGBIs. Staffing requirements, space limitations, evolving guidelines and best practices, and cancellations and rescheduling resulting from COVID-19 can also significantly increase wait times for a POC breath test.
Pro Forma Analysis for Point-of-Care Breath Testing
Many institutions and healthcare providers still utilize the POC device model despite the apparent drawbacks. POC breath testing involves upfront costs for the provider and revenue opportunities when tests are billed directly to the insurance provider or patient.
The GI practice bills the patient or their insurer directly. After reimbursement, there is generally a small profit to the practice associated with each test – which varies based on each patient insurance plan.
Since COVID-19, profits associated with in-office breath testing have decreased due to the increased overhead cost related to inflation, disinfection of devices, patient area, and equipment, as well as additional PPE necessary for each patient.
The amount of revenue a practice can generate from POC breath testing, and the ability to scale a POC breath testing model, is often restrictive due to staffing and equipment usage limitations.
In-Depth Look at At-Home Breath Testing
Compared to the two to three hours spent in the office completing a POC breath test, at-home breath testing allows patients to collect these samples from the comfort and safety of their own home and on their own time.
The at-home breath testing process is simple and efficient for both providers and patients. Breath test companies provide the test kits to the provider – in some cases at no cost – to allow the practice to send the kits home to their patients whom the provider feels are appropriate for a breath test. Alternatively, some breath test companies ship the at-home test kit directly to the patient.
After following the preparation instructions, the patient collects their breath samples and returns them directly to the laboratory for analysis using a pre-paid, pre-addressed shipping label provided in the test kit. The laboratory analyzes the breath samples and submits insurance claims on behalf of the patient to analyze their breath samples. Tests are typically analyzed and reported to the provider within one business day of sample receipt.
After the insurance claim is submitted for the analytical component of the clinical laboratory services and test results are sent to the practice, the provider may submit an insurance claim for the professional service after the provider has reviewed and interpreted the test result.
Most at-home breath test kits are FDA registered, CE marked, and ISO-13485 certified. However, it is essential to note that not all manufacturers follow these standards, so healthcare providers and patients should carefully choose a test kit.
In addition to kit manufacturing, it is also essential to consider how and where samples are analyzed. Healthcare providers should utilize test kits analyzed by experienced laboratory staff at a CLIA-certified laboratory, assuring optimal process quality, precise data accuracy, and timely results transmission.
Pro Forma Analysis for At-Home Breath Testing
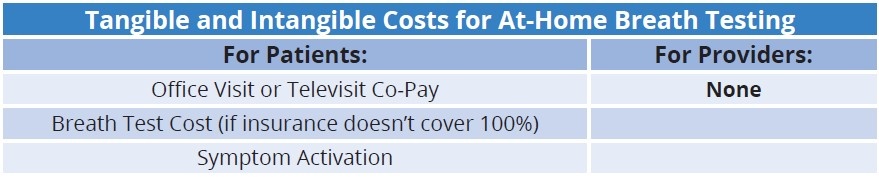
At-home breath testing provides a revenue opportunity without any cost – the provider is never responsible for upfront payments, nor does the provider have to incur expenses for laboratory equipment, consumables, and labor to administer the breath test.
When at-home breath test results are reported, they must be interpreted by each healthcare provider independently, who will use the laboratory values and data in conjunction with their other clinical information to make an informed medical determination and/or diagnosis for their patient. The attending physician who interprets and analyzes the test report is entitled to compensation for their expertise and services rendered, and such services are typically eligible for insurance reimbursement.
To obtain reimbursement, a provider and/or practice can submit an insurance claim using CPT code 91065 with a modifier of “26” to denote the professional component, written: “91065-26.” Healthcare providers and practicing receive a range of reimbursement for this CPT code, which is mostly dependent upon the insurance carrier, the specific patient plan, and the billing processes associated with each claim submission.
With no capacity or staffing limitations, revenue potential with the at-home breath testing model is scalable based on the size of the practice and needs of practice’s patient population.
Enhanced Patient Care + Optimized Revenue Model
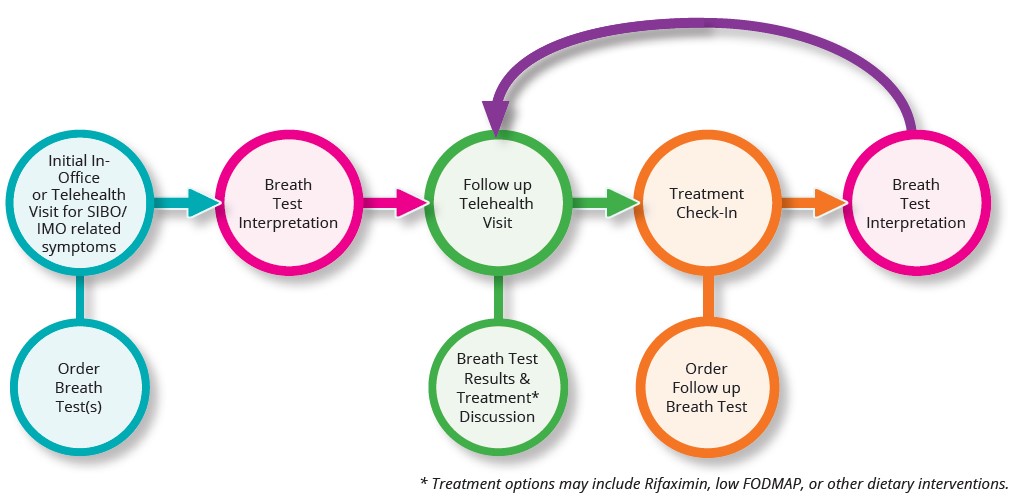
For patients complaining or presenting with symptoms that could lead to a SIBO, IMO, or a carbohydrate malabsorption diagnosis, the latest industry guidelines and best practices support an enhanced patient care model for diagnosis and treatment. This phased approach lowers the overall healthcare system costs and results in an optimized revenue model for providers due to the need for follow-up visits and diagnostic testing.
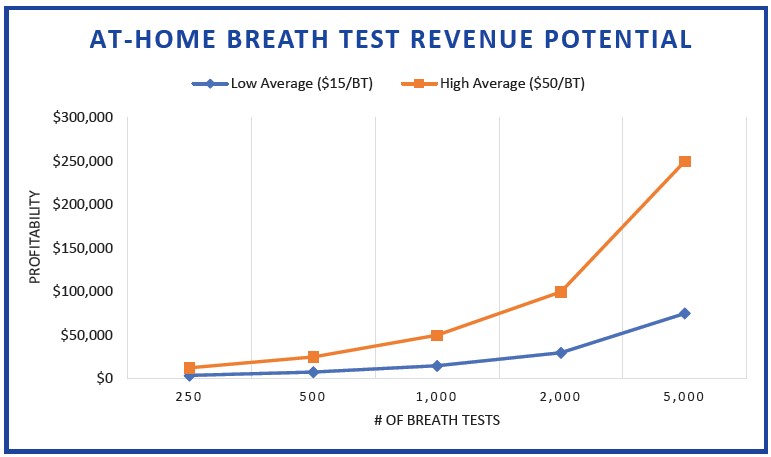
By utilizing at-home diagnostic breath testing as part of this enhanced patient care model, providers can exponentially increase breath test volume and revenue, with the average patient taking at least two breath tests during the diagnostic and treatment process. With no upfront costs and breath testing profitability between $15 (low average) and $50 (high average) per patient, the at-home breath test model is significantly more advantageous than the POC model with fixed profitability.
Conclusion
While the POC breath test model can provide varying degrees of profitability on a per-test basis, the overall level of profitability attainable for any provider or practice under the POC model itself is fixed. This is because POC breath testing is laborious, time-consuming, and limiting in terms of the number of overall breath tests able to be performed on-site at any one location. POC breath testing can also restrict a practice and individual providers from keeping up with patient demand or obtaining their desired turnaround time for their breath test results.
With the implementation of at-home breath testing, providers can immediately enhance the value of their practices both clinically and financially. Providers can eliminate the restrictive and costly process associated with POC breath testing while readily obtaining the clinical data necessary to guide patient care responsibly. Additionally, every breath test analyzed in the at-home model is 100% profitable for the practice with no upfront or added costs.
CDI’s at-home breath testing model also allows practices and providers to run an unrestricted quantity of breath tests while experiencing a 24-hour turnaround time on all tests. This is complemented by the ability of the provider or practice to generate revenue for its professional services associated with their interpretation of each test analyzed.
CDI is committed to using the most sophisticated analytical methods while also providing a seamless, cost-effective sample collection, transportation, and result-delivery process for our health care providers and their patients.